Compliant Pharma operations: LegalBook regulatory expertise
Legal departments of the pharmaceutical industry are under a lot of pressure to address compliance requirements, IP protection, and delicate contracts. Such challenges result in delays, compliance issues, and inefficiencies, which underlines the likelihood of new successfully approaches to work with the law.
Key Challenges
The sector is closely regulated by different agencies, including the FDA and the EMA in the developed world. Research shows that as many as 60% of compliance problems originate from issues related to contracts or their documentation. Deloitte’s insight is that such errors can lead to the grounding of products and massive penalties and are a risk to operational timeframes and revenues.
Pharmaceuticals is built on intellectual property, making it necessary to have well-drafted contracts prepared to protect that property. In contrast, clauses that touch on IP rights, patents, and/or licenses, and non-disclosure are seldom an exception that experience one or more iterations of negotiation. According to PwC, pharma contracts require a minimum of three to four rounds of iterations before finalizing contracts; besides, there are always various risks that have to deal with uncontrolled alterations.
pharma contracts commonly pertain to Research and development collaborations, manufacturers, distributors. According to McKinsey, the multiple stakeholder participation in the project causes the project to slow down by 30-40% in concluding the contract. This is besides slowing down timelines and challenging the launch of key projects and products.
It is crucial to certify that enough scrutiny applied to the partnership agreements so as to avoid issues to do with quality, regulatory, and IP. EY reports that outstanding areas of due diligence result in undocumented risks that directly influence business operations and reputation in as much as 20% of partnership deals.
Solutions By LegalBook
Auto Redlining in Legalbook automatically scans the document for all compliance issues like FDA and EMA provisions needed to be addressed. Using Legalbook, compliance incidents are highlighted and contracting non-compliance are pointed, shortening the duration taken to enter into contracts while contractual obligations are met effectively.
Legalbook informs the user of IP-related clause in real-time and consists of features such as Risk Reporting and Obligations Mapping. This helps avoid modifications by unauthorized personnel, reduce times of draft preparation and maintain protection of patent rights and information!
A particular value of Legalbook can be seen in the Obligations Tracking and the option of the Compliance Dashboard which allow the work to be done together. Contract management at speed and in real time avoids back-and-forth communications with multi-party approvals to speed deals.
Legalbook is here to ease the time-consuming process of due diligence through Metadata Extraction and Contract Summary tools. Management and Law firms employed in the pharmaceutical organizations can pull out required data, identify risks and avoid compliance with quality control measures, thus minimizing the risks associated with contract development life cycle and enriching company’s partnership.
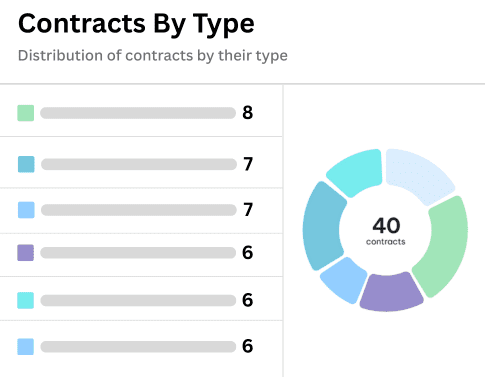
Request A Demo
See it yourself how AI can
transform your
contract management.
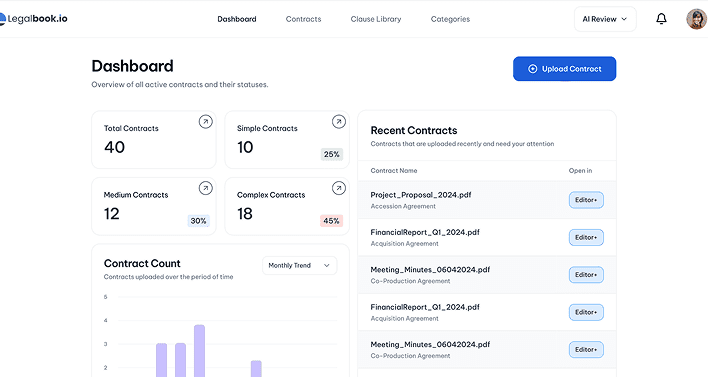
Read Our
Stay updated with the latest trends, tips, and insights in business analytics. Explore our curated
articles designed to empower your data-driven journey..